On March 30, 2024, Hengsai Bio announced that its premiere pipeline, KSD-101, was granted an Investigational New Drug (IND) license by the U.S. Food and Drug Administration (FDA), making it the first original dendritic cell vaccine (DC vaccine) product in China to receive an IND approval from the U.S. FDA.
Dr. Liu Huining, Founder and CEO of Hengsai Biotechnology, said, "We are pleased to share with you the good news that the safety and efficacy of KSD-101 has been preliminarily validated in the clinical IIT study, and now it is about to formally enter into the registered clinical phase, which is an important milestone in the company's R&D progress. This clinical approval is a high degree of recognition of our DC vaccine by overseas drug regulatory authorities, which demonstrates our innovative capability and international competitiveness in the field of immunotherapy. We are currently actively promoting the registration filing of KSD-101 in China and preparing for the construction of the production base."
About KSD-101
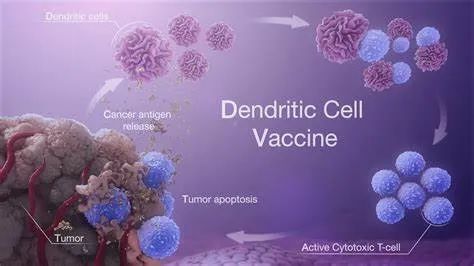
KSD-101 is a human monocyte-derived autologous dendritic cell vaccine loaded with EBV-associated tumor-like complex antigen. It is a tumor therapeutic vaccine prepared by inducing monocytes to transform into dendritic cells through cytokines, loading dendritic cells with tumor-like complex antigens and inducing them to become mature dendritic cells. After subcutaneous injection, it can activate EBV-specific CTL in the human body, thus realizing effective recognition and killing of tumor cells, with good effectiveness and safety.
About Hengsai Bio
Founded in 2018, Hengsai Bio is a scientific and technological innovative enterprise and national high-tech enterprise dedicated to the research, development and industrialization of dendritic cell vaccines (DC vaccines). The company has successfully constructed the Eco-DCVax platform, aiming to break down the technical barriers to industrialization and develop global First-in-Class high-quality DC vaccine products with independent property rights that are safe, efficient and convenient to administer. The developed product, therapeutic dendritic cell vaccine, provides a new approach and strategy for the treatment of relapsed and refractory tumors, chronic viral infections, autoimmune diseases and other diseases that are in urgent need of effective clinical treatments.
Hengsai Bio's R&D center and pilot plant in Shanghai Lingang Blue Bay officially came into operation in 2021, and a large-scale R&D/production base is under construction. The company has now established stable cooperative relationships with a number of domestic and foreign research institutions and tertiary hospitals, and is in the process of dual-reporting between China and the U.S. to carry out clinical research and technical cooperation in order to comprehensively verify the clinical safety and effectiveness of the DC vaccine.
 Hengsai Biotechnology
Hengsai Biotechnology


![DC Vaccine for Nasopharyngeal Carcinoma [Medium Swelling] Completes First Patient Enrollment for Dosing DC疫苗治疗鼻咽癌【中肿】完成首例患者入组给药](https://www.kousai.vip/wp-content/uploads/2025/02/文章封面-7-300x168.jpg)


