December 30, 2025Hengsai Bio's first pipeline of KSD-101 dendritic cell (DC) vaccine for EBV-related hematologic tumors was officially landed in Hainan Hospital of Ruijin Hospital affiliated with Shanghai Jiaotong University School of Medicine.. The project was declared and selected, evaluated by experts, verified at the production site and jointly approved by the Health Commission of Hainan Province, the Administration of the Lecheng Advance Zone and the Drug Administration.
KSD-101 is developed by Hengsai Biotechnology based on its self-developed "Eco-DC Vax" technology platform.The world's first broad-spectrum DC vaccine targeting EBV, as well as the first original DC vaccine in China to receive FDA IND approval, and the first DC vaccine product in China to receive FTD recognition... Individualized therapeutic vaccines are prepared by harvesting the patient's own mononuclear cells, inducing differentiation into dendritic cells in vitro, and loading them with EBV-associated specific complex antigens. Byhypodermic injectionAfter being infused back into the patient's body, it can precisely activate and train the patient's own immune system to recognize and kill tumor cells expressing EBV antigens and establish a long-lasting anti-tumor immune memory.
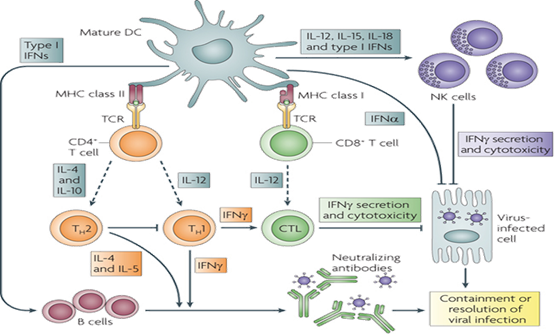
Tumor-killing mechanisms of the immune system in vivo
In the clinical study of KSD-101 monotherapy for the treatment of multiple lines of relapsed and refractory EBV-associated hematological tumors, the objective remission rate (ORR) was as high as 91.67%, of which the complete remission rate (CR) was 83.33%, and the disease control rate (DCR) was 100%; meanwhile, it demonstrated a durable response and a good safety profile, and the adverse reactions were mainly injection site reactions and fever and all of them are below grade 2.
The landing of KSD-101 in Hainan Hospital of Ruijin Hospital will bring a brand new therapeutic hope for patients with relapsed and refractory EBV-positive related hematological tumors. Currently, the team is gradually promoting other indications of DC vaccine therapy in the LeCheng Advanced Zone, in order to provide more tumor patients with innovative treatment options that are safe, efficient and convenient to administer.
About Kousai
Founded in 2018, Kousai Bio is a scientific and technological innovative enterprise and national high-tech enterprise dedicated to the research, development and industrialization of dendritic cell vaccines (DC vaccines). The company has successfully constructed the Eco-DCVax platform, aiming to break down the technical barriers to industrialization and develop global First-in-Class high-quality DC vaccine products with independent property rights that are safe, efficient and convenient to administer. The developed product, therapeutic dendritic cell vaccine, provides a new approach and strategy for the treatment of relapsed and refractory tumors, chronic viral infections, autoimmune diseases and other diseases that are in urgent need of effective clinical treatments.
Relying on the self-constructed Eco-DC Vax platform, the company systematically integrates the four modules of antigen prediction, immunological validation, process optimization and quality control, which enables "one drug, multiple targets" and platform scalability. At present, we have a comprehensive R&D and production base with a floor area of 6,300 square meters and a production capacity of about 5,000 servings/year in Lingang New Area of Shanghai FTZ, equipped with the industry's top facilities, and possessing the hard power from scientific research to industrialization.
 Hengsai Biotechnology
Hengsai Biotechnology